What type of bond do magnesium and sulfur form?
- Number Of Electrons In Magnesium
- Number Of Electrons In Magnesium Cation
- Number Of Electrons In Magnesium Atom
More realistically, each magnesium atom has 12 protons in the nucleus compared with sodium's 11. In both cases, the nucleus is screened from the delocalised electrons by the same number of inner electrons - the 10 electrons in the 1s 2 2s 2 2p 6 orbitals. Magnesium has atomic number 12. Therefore, an atom of Mg has twelve electrons, with ground state configuration: 1s2 2s2 2p6 3s2. The valence electrons are the ones in the 3s subshell. Magnesium forms Mg^2+ ions when reacting with non-metals. Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n 2) electrons.
1 Answer
Explanation:
Magnesium,
Number Of Electrons In Magnesium
On the other hand, sulfur,
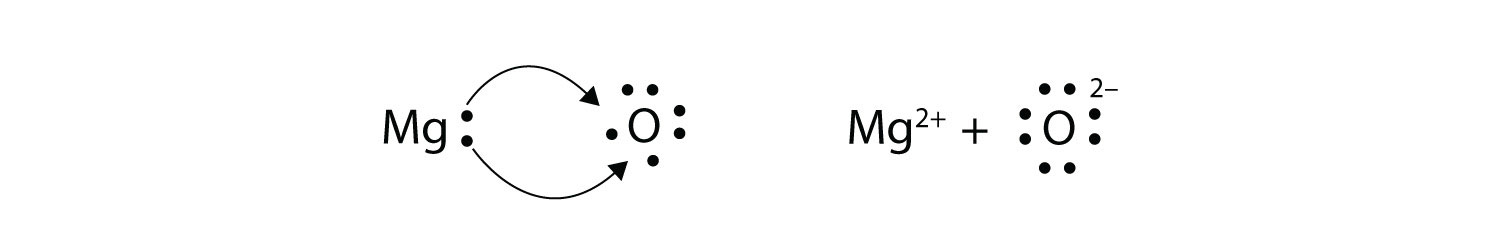

Number Of Electrons In Magnesium Cation
As you know, chemical reactivity is governed by an atom's 'desire' to have a stable electron configuration, that is, to have eight electrons in its outermost shell
In this case, magnesium can complete its octet by giving up those two valence electrons, becoming the magnesium cation.
Sulfur, which only needs two electrons to complete it octet, will pick up the two electrons coming from magnesium, becoming the sulfide anion,

The electrostatic force of attraction will then bring the magnesium cations and the sulfur anions together
Number Of Electrons In Magnesium Atom
Related questions
